Background: von Willebrand disease (VWD) is characterized by a deficiency or reduction of activity in von Willebrand factor (VWF). As VWF has a role in stabilizing coagulation factor VIII (FVIII), VWD patients often suffer from secondary FVIII deficiency. Current guidelines recommend that VWD patients with a history of severe and frequent bleeds should use long-term prophylaxis with factor replacement therapy. The WIL-31 study demonstrated the efficacy of prophylaxis with a plasma-derived VWF/factor VIII concentrate containing VWF and FVIII in a 1:1 activity ratio (pdVWF/FVIII; wilate ®) in adults and children with VWD. The primary endpoint was met, showing a decrease of 84% in the mean total annualized bleeding rate (ABR). As elevated levels of FVIII are associated with an increased risk for thrombosis, it is important to determine whether repeated dosing with pdVWF/FVIII leads to accumulation of FVIII over time.
Aims: To investigate the activity levels of VWF and FVIII in patients with VWD receiving regular prophylaxis with pdVWF/FVIII.
Methods: WIL-31 (NCT04052698) was a prospective, non-controlled, international, multicenter phase 3 trial that enrolled male/female patients, aged ≥6 years old with VWD type 1 (VWF:RCo <30 IU/dL), type 2 (except 2N) or type 3. Prior to entering the WIL-31 study, all patients had received on-demand treatment with a pdVWF/FVIII concentrate during a 6-month, prospective, observational, run-in study (WIL-29). Patients in WIL-31 received regular pdVWF/FVIII prophylaxis 2-3 times per week at a dose of 20-40 IU/kg for 12 months. VWF activity (VWF:RCo) and FVIII activity (measured by the chromogenic [Chr] and one-stage [OS] assays) were performed at baseline and throughout the study. Full pharmacokinetic (PK) analyses were performed in all patients aged 6-16 years, a single dose of 60 ± 10 IU/kg pdVWF/FVIII was administered. Samples were collected at up to 1 hour prior to injection and then at multiple time points post-injection (1-72 hours). Safety, including the occurrence of thrombotic events, was monitored throughout the study.
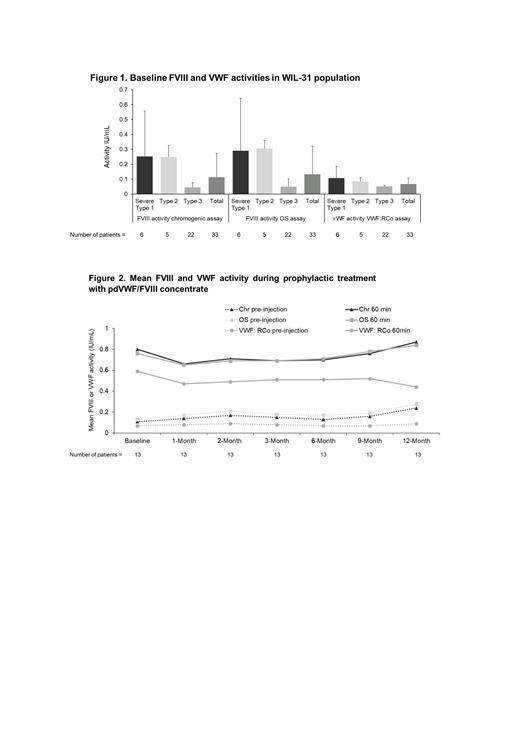
Results: The overall study population included 33 patients, with a median age of 18 years consisting of 9 (27.3%) patients aged 6-11 years, 6 (18.2%) patients aged 12-16 years, and 18 (54.5%) patients aged ≥17 years. Six (18.2%) patients had severe type 1 VWD, 5 (15.2%) patients had type 2, and 22 (66.7%) patients had type 3. Baseline FVIII and VWF activities in WIL-31 were similar regardless of age group, and as expected were lower in patients with type 3 VWD compared with severe type 1 and type 2 (Figure 1). There was a negative correlation of baseline FVIII and VWF activity with the number of spontaneous bleeding episodes (BEs) during prophylaxis. Patients who had no spontaneous BEs during prophylaxis had higher baseline FVIII and VWF levels compared with those who had spontaneous BEs. Mean peak and trough FVIII and VWF activity levels remained consistent during the study and there was no accumulation of either factor over the 12-month prophylaxis period (Figure 2). Full PK assessments were performed according to protocol in 13 patients aged 6-16 years. The mean ± standard deviation in vivo half-life was 16.1±6.7, 16.2±7.7, and 9.8±7.7 hours, for FVIII Chr, FVIII OS, and VWF:RCo, respectively. No thrombotic events were observed.
Conclusion: No accumulation of FVIII or VWF in the plasma was reported in 33 patients who received regular prophylaxis with pdVWF/FVIII for 12 months, regardless of VWD type and age.
OffLabel Disclosure:
Khayat:LFB: Honoraria; CSL Behring: Honoraria; Octapharma: Honoraria. Sidonio:Guardian Therapeutics: Honoraria; Bayer: Honoraria; Novo Nordisk: Honoraria; UniQure: Honoraria; Biomarin: Honoraria; Pfizer: Honoraria; Spark: Honoraria; Genentech: Honoraria, Research Funding; Octapharma: Honoraria, Research Funding; Takeda: Honoraria, Research Funding.
This study is investigating the use of a VWF / FVIII concentrate for the prevention of bleeds in patients with von Willebrand disease of any type

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal